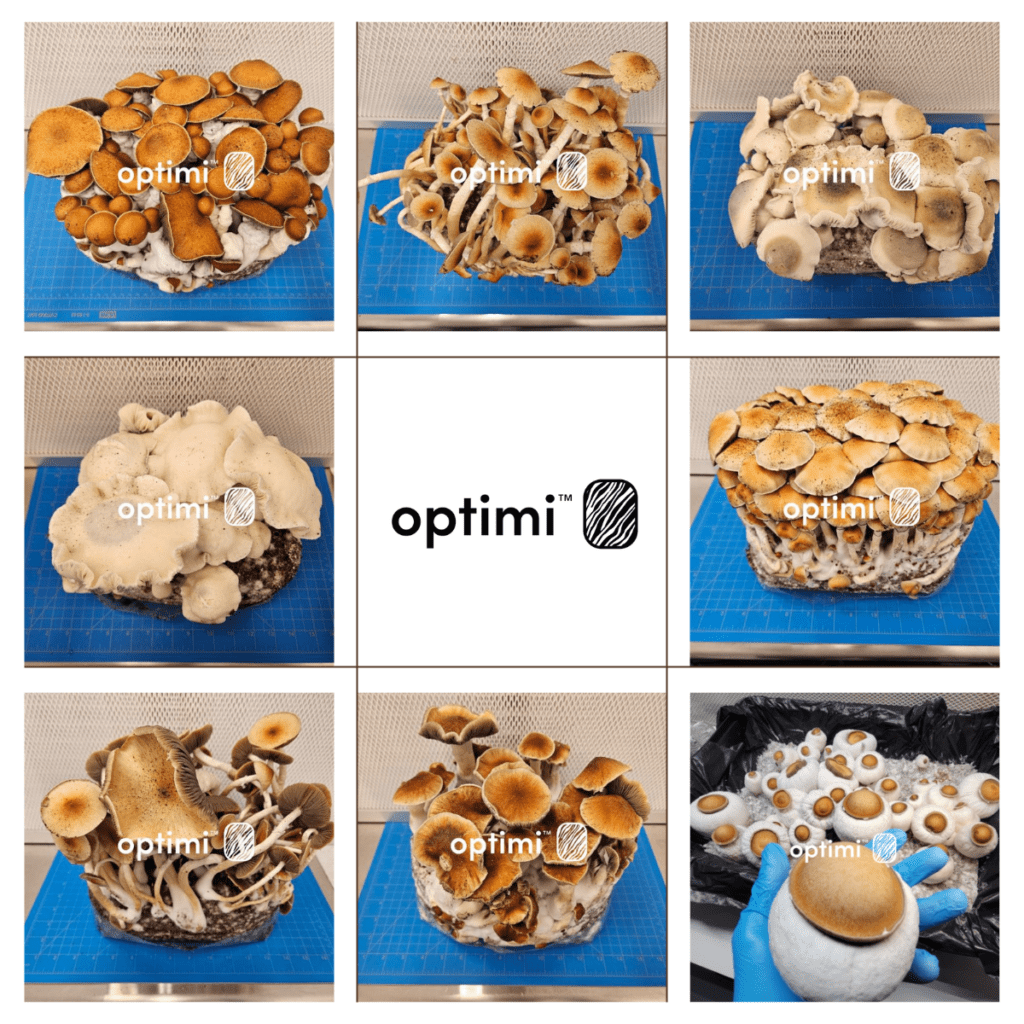
Vancouver, British Columbia, November 28, 2023 – Optimi Health Corp. (CSE: OPTI) (OTCQX: OPTHF) (FRA: 8BN), a leading end-to-end drug researcher and formulator licensed by Health Canada, has reached a significant milestone in its mission. The company is proud to announce the successful harvest of eight new natural Psilocybin genetics, strengthening its position as a pioneering force in the psychedelic research and formulation space.
Demonstrating its commitment to innovation and research, Optimi has cultivated these new Psilocybin-containing mushroom strains with the aim that they will play a pivotal role in the development of psilocybin extracts and novel drug candidates.
Optimi also announces the advancement of its full spectrum, GMP extract quality program. The Company has successfully completed process validation for the natural psilocybin drug candidate, and the stability testing according to ICH guidelines is ongoing. With over three months of accelerated data, Optimi can confirm the product is able to maintain potency and other quality attributes for the duration of clinical trials.
CEO William (Bill) Ciprick stated, “Our production of a scalable GMP compliant extract is a crucial step towards facilitating further research and maintaining our position as a reliable and innovative partner in the development of psilocybin-based therapies.”
While Optimi continues to develop its product offerings, it remains focused on fulfilling supply orders, conducting tests, and analyzing the recent milestone harvest. The company is pleased to announce the availability of GMP Psilocybin API for encapsulation into standardized dosages or for sale to other groups as a raw material for their formulations. This marks a significant step in expanding the company’s reach and impact within the psychedelic research community.
Dane Stevens, co-founder, and director, envisions Optimi’s role as a key contributor to this evolving landscape:
“Optimi continues to focus its research and development efforts on expanding its knowledge base and product offerings, with the aim of playing an integral role in the global psilocybin market. We are well-positioned to be a trusted partner for drug developers and advanced research programs around the world.”
The new genetic strains were cultivated by Optimi’s Cultivation Manager, Scott Marshall, who oversees the company’s large-scale operations, genetic advancement, and the stabilization of both novel and existing varieties of Psilocybe cubensis with remarkable proficiency.
Pictures of Optimi’s newest genetics strains can be found here.
**All photos provided by Optimi are subject to copyright protection. Reproduction or use for commercial purposes without explicit permission from Optimi is prohibited. Users are encouraged to contact Optimi for licensing inquiries and permissions regarding photo reproduction.**
For media inquiries, please contact Michael Kydd at:
pr@optimihealth.ca
902.880.6121
For investor inquiries, please contact us at:
investors@optimihealth.ca
www.optimihealth.ca